what is the nucleus of an atom
The number of protons and electrons are the same in atom and that is. The protons and neutrons form a very small dense core known as the nucleus.
 |
| 3 5 The Atomic Nucleus Chemistry Libretexts |
It is composed of protons which have a positive charge and neutrons which have no charge.
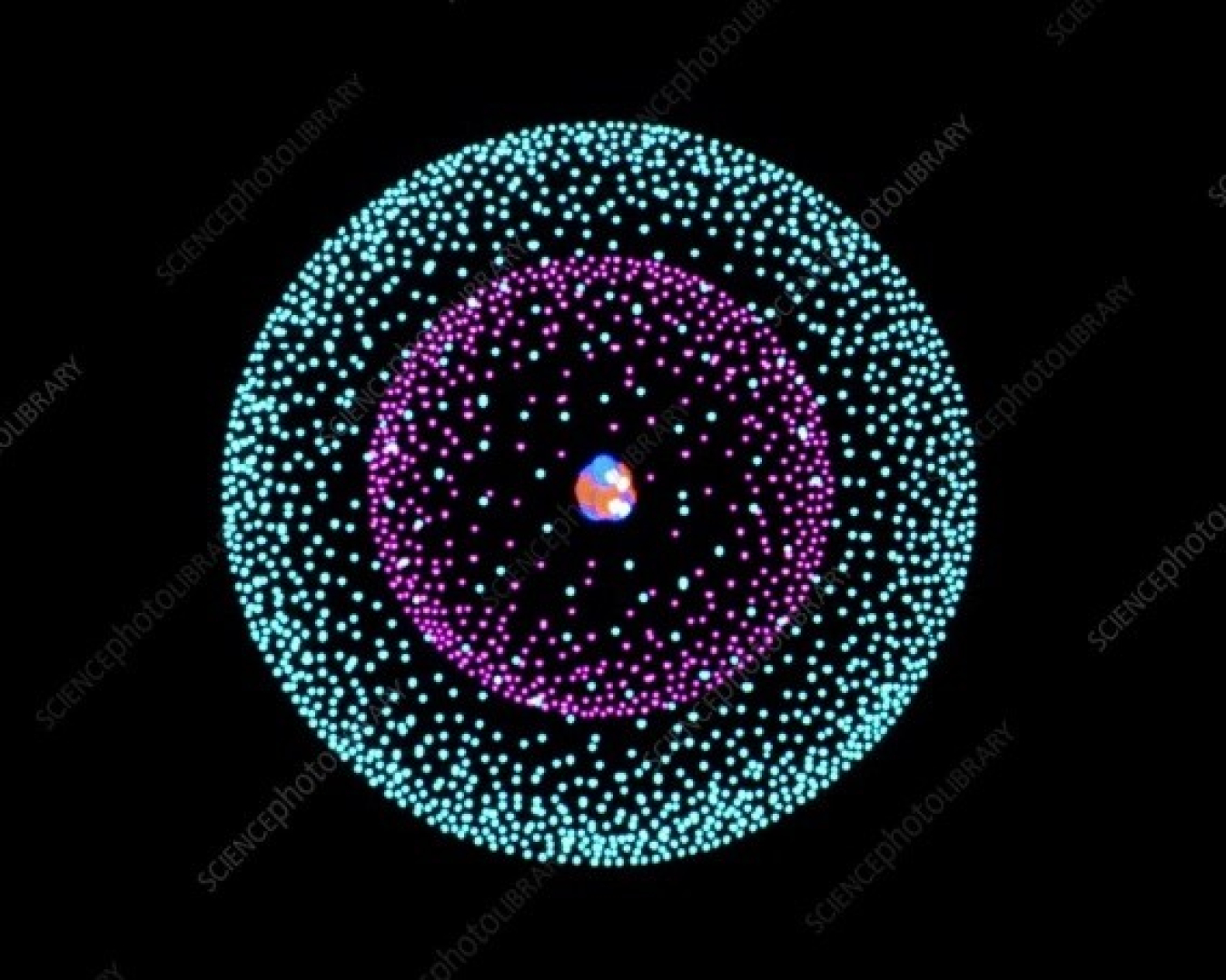
. The atomic nucleus is the small dense region consisting of protons and neutrons at the center of an atom discovered in 1911 by Ernest Rutherford based on the 1909 GeigerMarsden gold foil experiment. Virtually all the mass of an atom resides in its. An atomic nuclei consist of positively charged particles protons and uncharged particles neutrons. Which are in the nucleus of the atom and have mass.
Nucleus of an atom. The nucleus of an atom is the location where protons and neutrons are found. The number of protons in the nucleus known as the atomic number primarily determines where. Electrons move in orbits.
Therefore most of the mass of an atom is contained in its nucleus. After the discovery of the neutron in 1932 models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. The atomic nucleus is the small dense region consisting of protons and neutrons at the center of an atom discovered in 1911 by Ernest Rutherford based on the 1909. The atomic nucleus is the small dense region consisting of protons and neutrons at the center of an atom discovered in 1911 by Ernest Rutherford based on the 1909.
An atom is composed of a positively charged nucleus with a cloud of negatively charged electrons surround. Protons and neutrons are in turn made up of particles called. The nucleus is the positively charged centre of an atom and contains most of its mass. The protons and neutrons form a very small dense core known as the nucleus.
Electrons move in orbits. The nucleus is present in the centre of the atom and is surrounded by the extranuclear portions. Therefore most of the mass of an atom is contained in its nucleus. The nucleus of an atom is the central region of an atom that contains the majority of its mass.
The nucleus is a collection of particles called protons which are positively charged and neutrons which are electrically neutral. The nucleus or center of an atom is made up of protons and neutrons. Protons and neutrons are in turn made up. The nucleus is a collection of particles called protons which are positively charged and neutrons which are electrically neutral.
The electrons are found in a region of space called the electron cloud. The atomic nucleus is the small dense region consisting of protons and neutrons at the center of an atom discovered in 1911 by Ernest Rutherford based on the 1909 GeigerMarsden gold foil. In the given case the atomic number of the given atom is 15 hence its nucleus contains 15 protons. Rutherfords scattering of alpha particles experiment revealed that the nucleus of.
Protons and neutrons reside in the nucleus and are together called. The nucleus or center of an atom is made up of protons and neutrons. An atom is composed of a positively charged nucleus and.
/GettyImages-1017116892-917f9457f2bc4e4cbca2827b9d0a8966.jpg) |
| Why Protons And Neutrons Stick Together In The Nucleus |
 |
| Which Two Subatomic Particles Are Located In The Nucleus Of An Atom Brainly Com |
 |
| Nucleus Of The Atom Ck 12 Foundation |
 |
| New Picture Of Atomic Nucleus Emerges Argonne National Laboratory |
 |
| The Structure Of The Atom Boundless Chemistry Course Hero |
Posting Komentar untuk "what is the nucleus of an atom"